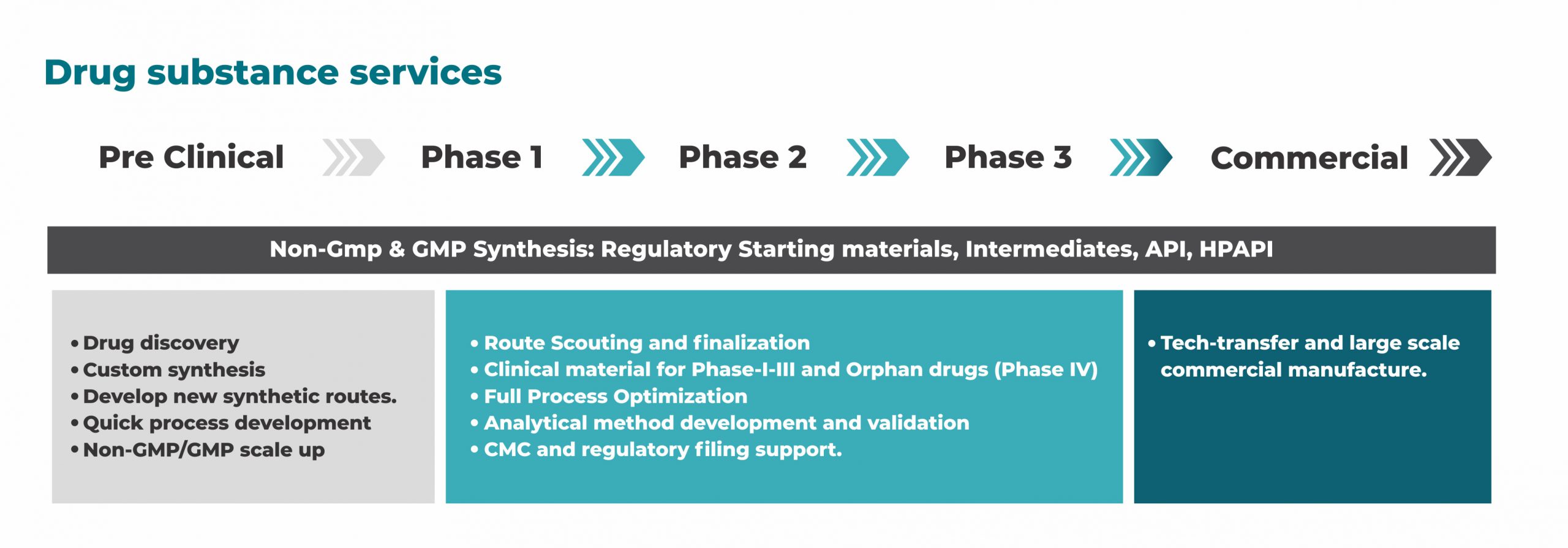
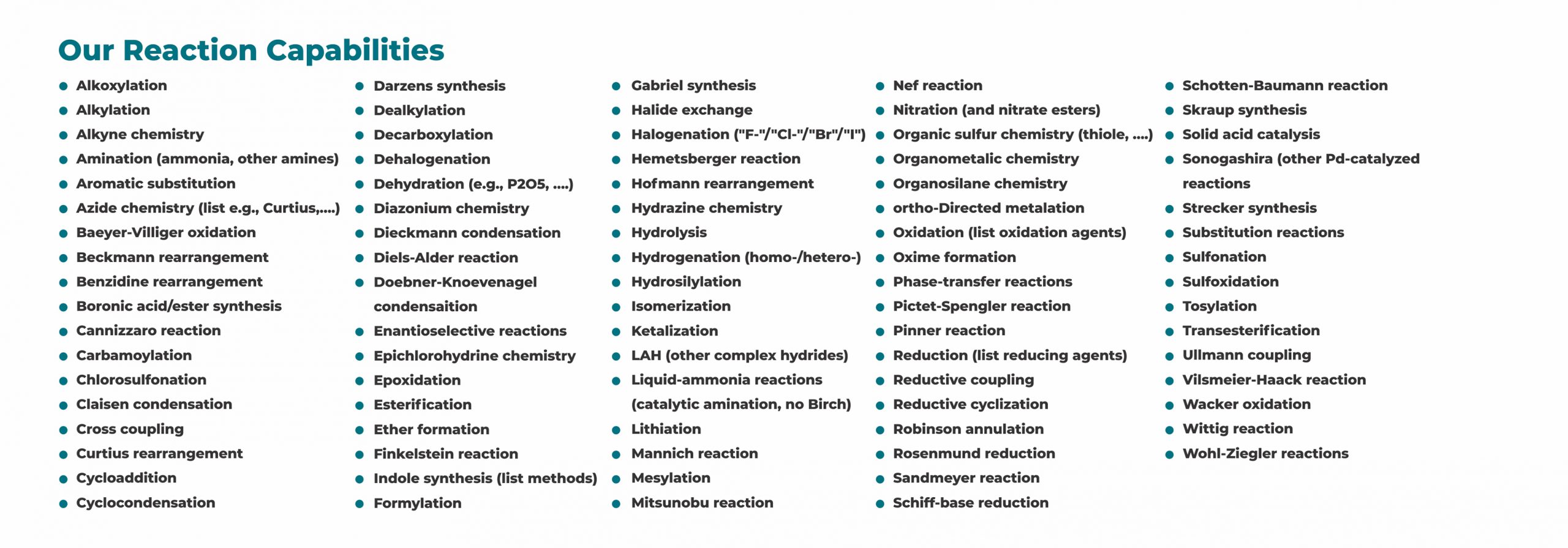
Exhaustive literature search combined with our experience on synthetic methodology enables us to scout synthetic route involving lesser steps with improved yields and feasible purification. Redox provides following economic, safe and green process chemistry services:
- small scale GMP – Orphan Drugs
- Clinical stage manufacturing – Phase I, Phase II and Phase III
- cGMP API, Advanced Intermediates and RSM
- Non-GMP Kg to MT quantities
- Bulk Scale Process Optimization and Process Development
- Develop new synthetic routes
- Evaluate process safety
- Manufacture compounds, regulated starting materials and intermediates as per GMP guidelines
- Catalyst Screening
- Kilo lab manufacturing
- Pilot scale batch
- Validation batch
- Drug substance